Phase 2: Evaluating Effectiveness and Identifying Side Effects
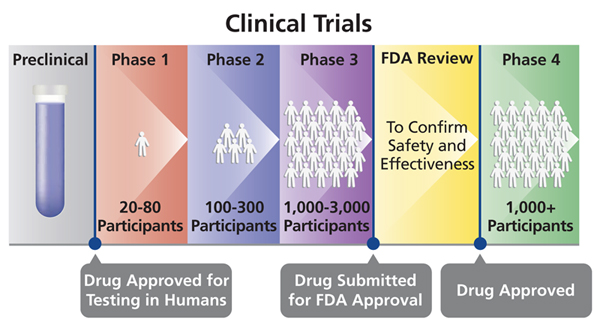
Building on the safety data from Phase 1, Phase 2 trials involve a larger group of participants (100-300) who represent the target population for the vaccine. In this stage, researchers assess the vaccine’s effectiveness in generating an immune response and identify any additional side effects that may emerge.
Phase 3: Large-Scale Efficacy Testing and Monitoring
Phase 3 trials are the largest and most extensive phase of clinical research, involving thousands of participants across multiple geographic locations.
The primary goal of this phase is to confirm the vaccine’s efficacy in preventing the targeted disease.
Researchers also continue to monitor for potential side effects and assess their frequency and severity.
These trials provide robust evidence of the vaccine’s real-world effectiveness and safety before regulatory approval.
Phase 4: Post-Market Surveillance
Even after a vaccine receives regulatory approval and becomes available to the public, monitoring continues. This phase involves ongoing surveillance to identify any rare or long-term side effects that may not have been detected during clinical trials.
Ethical Considerations in Vaccine Trials
Vaccine clinical trials are conducted under strict ethical guidelines to ensure the safety and well-being of all participants. These guidelines include:
- Informed Consent: Participants must fully understand the risks and benefits of participating in the trial and voluntarily consent to participate.
- Data Confidentiality: Participant data is collected and analyzed anonymously to protect their privacy.
- Independent Review Boards: Ethical review boards comprised of independent experts review and approve all aspects of the trial to ensure its ethical conduct.
The Importance of Vaccine Clinical Trials
Vaccine clinical trials are essential for several reasons:
- Safety: Trials rigorously evaluate the safety of vaccines and identify potential adverse effects.
- Efficacy: They provide robust evidence of a vaccine’s ability to prevent disease.
- Target Population Specificity: Trials tailor vaccine development to specific populations, ensuring their effectiveness and safety.
- Public Health Impact: By accelerating the development and approval of safe and effective vaccines, clinical trials contribute significantly to global health efforts.
FAQs about Vaccine Clinical Trials
1. How long do vaccine clinical trials take?
Vaccine clinical trials can take several years to complete, ranging from 5 to 10 years or even longer.
2. Who can participate in vaccine clinical trials?
Eligibility criteria for clinical trials vary depending on the vaccine being tested.
Typically, trials seek participants who represent the target population for the vaccine.
3. Are vaccine clinical trials safe?
Vaccine clinical trials are conducted under strict safety protocols to minimize risks to participants. Side effects are closely monitored, and participants receive appropriate medical care if needed.
4. Do participants in vaccine clinical trials receive the vaccine free of charge?
Yes, participants in vaccine clinical trials typically receive the vaccine free of charge, along with other support such as medical monitoring and compensation for their time and expenses.
5. What motivates people to participate in vaccine clinical trials?
People choose to participate in vaccine clinical trials for various reasons:
- Contributing to scientific advancement: They want to play a role in developing life-saving vaccines.
- Potential health benefits: They may be willing to take a risk for the benefit they might receive from the vaccine.
- Contribution to public health: They understand the importance of vaccines for protecting themselves and others.
Conclusion
Vaccine clinical trials are a cornerstone of public health, safeguarding the safety and effectiveness of the vaccines that protect us from devastating diseases. While the process is complex and demanding, the benefits of eradicating and controlling infectious diseases far outweigh the challenges. By understanding the intricate stages of vaccine clinical trials, we can appreciate the diligent work of scientists, researchers, and healthcare professionals who tirelessly dedicate themselves to developing and delivering life-saving vaccines.
Closure
Thus, we hope this article has provided valuable insights into Clinical trials for vaccines. We thank you for taking the time to read this article. See you in our next article!